In the era of globalization, the balance between the vigorous development of high-tech industries and safety and compliance is increasingly valued. Recently, the Ministry of Public Security and relevant departments jointly issued an announcement on the optimization of service management measures for some specific products containing γ-butyrolactone, which reflects this balance effort. This move aims to ensure the smoothness of the industry supply chain, while ensuring the safety and compliance of related products, and providing more efficient and convenient services for high-tech industries. The following is a detailed interpretation of the announcement:
1. Background and Significance
Due to its special properties, γ-butyrolactone was included in the "Catalogue of Dangerous Chemicals that are Easily Used to Make Narcotic Drugs" in 2021. The import and export of products containing this chemical ingredient requires a relevant license. Although this regulation strengthens safety supervision, it has brought certain challenges to the supply chain of high-tech industries. To this end, the Ministry of Public Security and other departments have decided to implement optimized supervision measures for some specific products containing γ-butyrolactone to ensure the stability of the industrial supply chain and the development of high-tech industries.
2. Specific optimization measures
This announcement clarifies the following optimized regulatory measures:
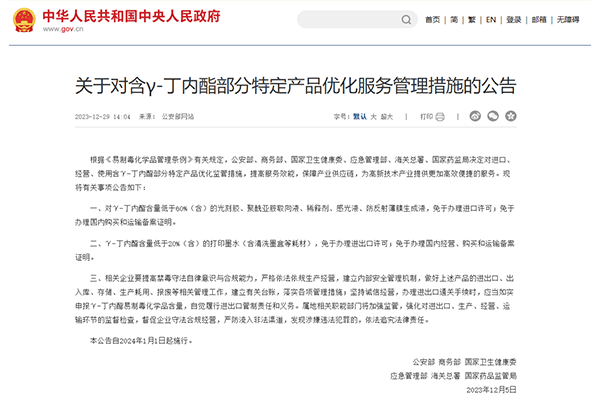
1. No import license required
Photoresists, polyimide alignment liquids, diluents, photosensitive liquids, and anti-reflective film forming liquids with a γ-butyrolactone content of less than 60% (inclusive) are exempt from the requirement for import licenses.
2. No need for domestic purchase and transportation registration certificate
The above-mentioned products are exempt from obtaining domestic purchase and transportation registration certificates, thus reducing the operational burden on enterprises.
3. Printing ink and related consumables
Printing inks and related consumables with a γ-butyrolactone content of less than 20% (inclusive) are exempt from obtaining import and export licenses and domestic operation, purchase, and transportation registration certificates.
III. Enterprise Compliance Requirements
This announcement emphasizes that relevant companies need to improve their compliance capabilities and self-discipline awareness and strictly conduct production and operations in accordance with laws and regulations. Specific requirements include:
1. Safety management mechanism
Establish an internal safety management mechanism to ensure the compliance of product use and storage, as well as related warehousing, production consumption and scrap management.
2. Integrity management
When going through import and export customs clearance procedures, the γ-butyrolactone content should be declared truthfully and the import and export control responsibilities and obligations should be fulfilled.
3. Strengthened supervision
The relevant local functional departments will strengthen supervision, and conduct inspections on import and export, production, operation, transportation and other links to ensure that enterprises operate in compliance with laws and regulations.
This announcement will be officially implemented on January 1, 2024. It is both an opportunity and a challenge for enterprises in high-tech industries, especially the electronics manufacturing industry. Enterprises need to strengthen internal management to ensure compliance with new regulatory requirements, while also enjoying the convenience brought by the policy. In this way, the government not only ensures the safety and compliance of the industry, but also supports the efficient development of the industry, achieving a win-win situation of safety and development.


 Follow customer service WeChat
Follow customer service WeChat